Syneos Health R&D Advisory EU CTR Readiness
Are You EU CTR Ready?
The European Union Clinical Trials Regulation (EU CTR) is set to radically change the clinical trial landscape across Europe. The new Clinical Trial Information System (CTIS) officially went live on 31st January 2022, marking the start of a three-year transition period during which time companies must implement updated processes and assess systems to enable compliance and effective interfacing with the CTIS.
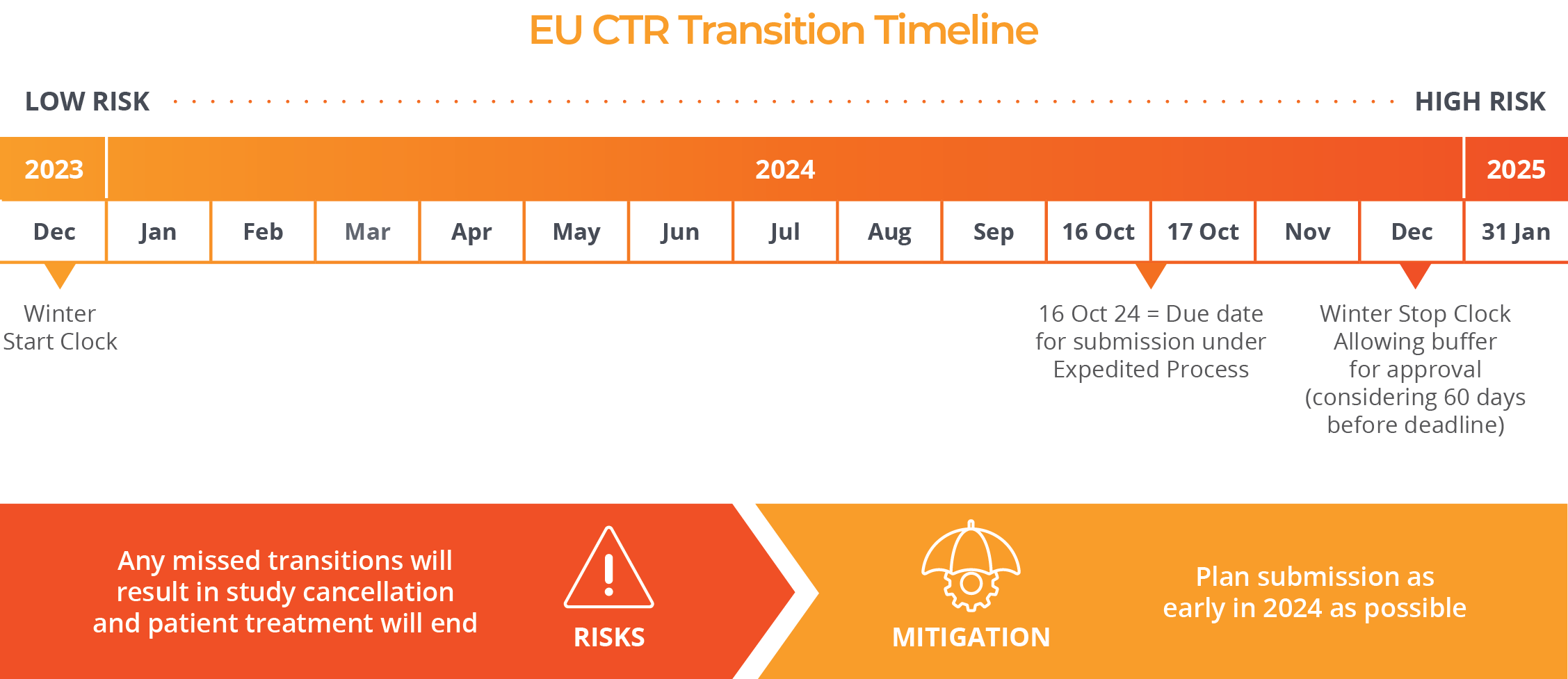
In order to transition to these new regulations, sponsors will need to submit trials before the end of the transition period on 30 January 2025. After that date, a transition will no longer be possible and a new clinical trial application under the new CTR will be required.
Until the middle of October of 2024, an administrative transition process published by the Clinical Trials Coordination Group (CTCG) is available with minimum requirements and short deadlines.
Remember, if your trial is running in EU/EEA and has at least one active site* beyond 31 January 2025, it is MANDATORY to switch the trial to the new EU regulation.
*Active site means that if the last visit last subject (LVLS) or any other trial specific intervention with the subject as specified in the protocol took place before this date, the trial does not need to be transitioned.
If there are no active sites in EU/EEA but the End of Trial has not yet been notified, the trial should not be transitioned.
Start immediately to prepare your transition.
The goal of the EU CTR is to create an environment which is favorable for the conduct of clinical trials in the EU, with the highest safety standards for participants and increased transparency of trial information. To support this aim, the regulation has introduced new requirements which sponsor companies must be aware of in order to enable operational readiness in line with the changes mandated by the regulation.

The transition application is a complex procedure and includes submission using the CTR-CTIS portal of the most recently approved CTD documentation in all member states concerned. The aim of this application is for you to continue to run your clinical trial beyond 30 January 2025 without discontinuation.
Syneos Health is uniquely positioned to expedite your CTR transition journey. We have a strong, established EU regulatory framework. We also have a proven track record demonstrating our knowledge, actionable insights and quality in multiple therapeutic areas, types of products, countries and trial design applications.
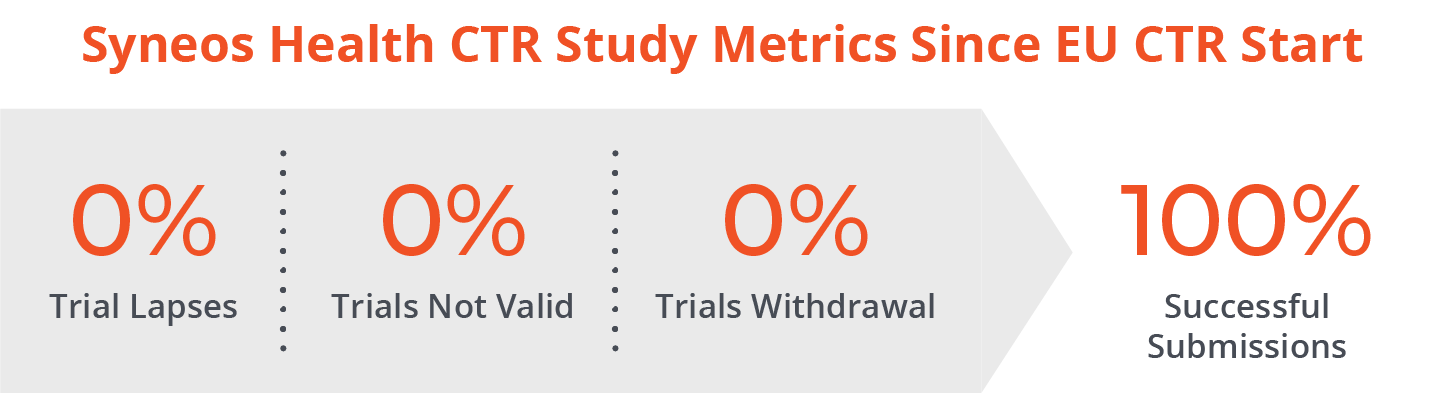
The Syneos Health integrated EU CTR offering can provide end-to-end support to sponsors irrespective of their readiness. We can support in preparing you to be ready to conduct trials under EU CTR and your ongoing delivery of clinical trials under the EU CTR. We are already supporting our customers with this.

