Life Sciences Consulting
Raising the bar in an industry where good enough never is
Born from a higher standard, Syneos Health Consulting was established with the understanding that traditional consulting approaches needed a sharper, more nuanced lens to address the unique challenges of the pharmaceutical industry. Backed by a 30-year legacy beginning with Campbell Alliance and integrating the expertise from Kinapse and global talent along the way, we continue to redefine healthcare consulting.
We help clients make decisions with confidence, de-risking decision making while maximizing outcomes. With direct access to physicians on the cutting edge and commercial experts, we bridge the gap between clinical reality and market success, ensuring every choice is backed by those shaping the future.
Optimizing assets, portfolios and organizations through data-driven decision support across the lifecycle
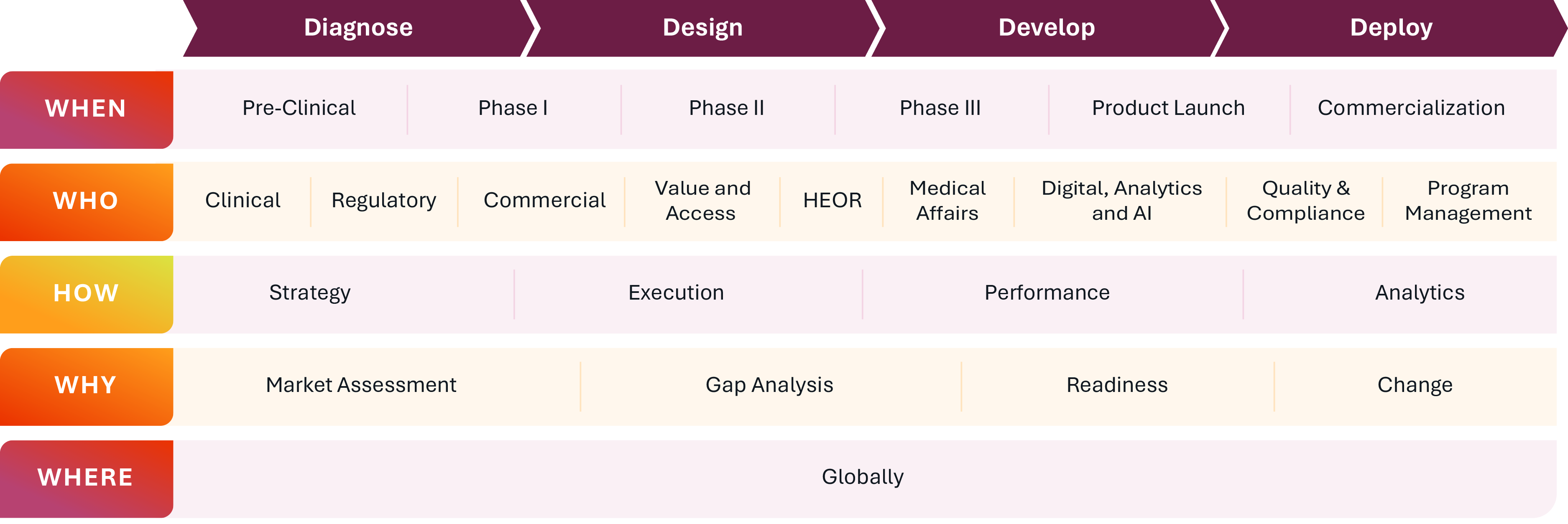

We bring cross-functional expertise, data-driven insights, and commercial
acumen to address the critical challenges biopharma organizations face:
The R&D Advisory group provides deep expertise across clinical, quality, regulatory, data management, technology, and pharmacovigilance (PV) from pre-clinical to post-market.
With a team of 90+ dedicated R&D consultants and subject matter experts, we help biopharma companies navigate the complexities of drug development, optimize operations, and accelerate clinical timelines. Our expertise spans operating model design, technology strategy, process optimization, and regulatory compliance.
We leverage our global network of industry experts and clinical partners to provide insights that drive transformational results — such as reducing clinical development timelines, streamlining costs, and improving quality to help clients maximize their R&D investments.
The Value and Access Advisory Group helps clients navigate the shifting market landscape, create and communicate value, and optimize access. With deep expertise in navigating the changing face of commercialization, our Market Access Consulting Group understands that every stakeholder has a unique definition of value, and how those definitions and needs impact brands throughout the product lifecycle.
Our team focuses on supporting clients of sizes navigate the changing face of commercialization. We approach market access questions with decades of experience, cross-functional expertise, and unparalleled stakeholder insight. By leveraging our diverse expertise, collaborative approach, and flexible solutions, we can help you successfully navigate market access barriers before and after launch, through LOE.
Health Economics and Outcomes Research (HEOR) plays a crucial role in reimbursement decisions by connecting clinical outcomes with economic impact, ensuring alignment with the priorities of the healthcare systems.
The HEOR team collaborates with you to develop tailored solutions that meet your product's needs throughout its lifecycle. To ensure successful product development and commercialization, it is essential that timely and relevant evidence is available to the key stakeholders. We offer a wide range of HEOR services, combining technical expertise with strategic insights including: literature review and synthesis, economic modeling, database analysis, reimbursement dossiers, evidence dissemination, and HEOR/ HTA strategy.
Given the competitive and capital-intensive nature of the pharma and biotech business development environment, companies face pressure to make strategic decisions on where to focus, how to grow, and which assets to prioritize or partner. In a landscape marked by evolving market dynamics, regulatory complexities, and a constant need for innovation, securing the right partnerships and maximizing asset value are critical for long-term success.
Our team helps biopharma companies and investors navigate these choices by evaluating strategic options that align with corporate objectives and aim to maximize asset value. We assist clients in defining a compelling story that resonates with potential partners or investors, grounded in market assessments, forecast modeling, and business case development. By investing in these foundational strategies, clients are positioned to unlock long-term value, both on the sell-side and buy-side.
With experience spanning early pipeline through late-stage lifecycle management, we’ve advised top global biopharma companies across geographies and therapeutic areas. Our team has completed 500+ portfolio, licensing, M&A, and transaction-focused projects and over 200 disease area assessments in the past five years. Recognized as thought leaders in dealmaking, we’ve published 20+ articles and presented at biotech conferences for eight consecutive years.
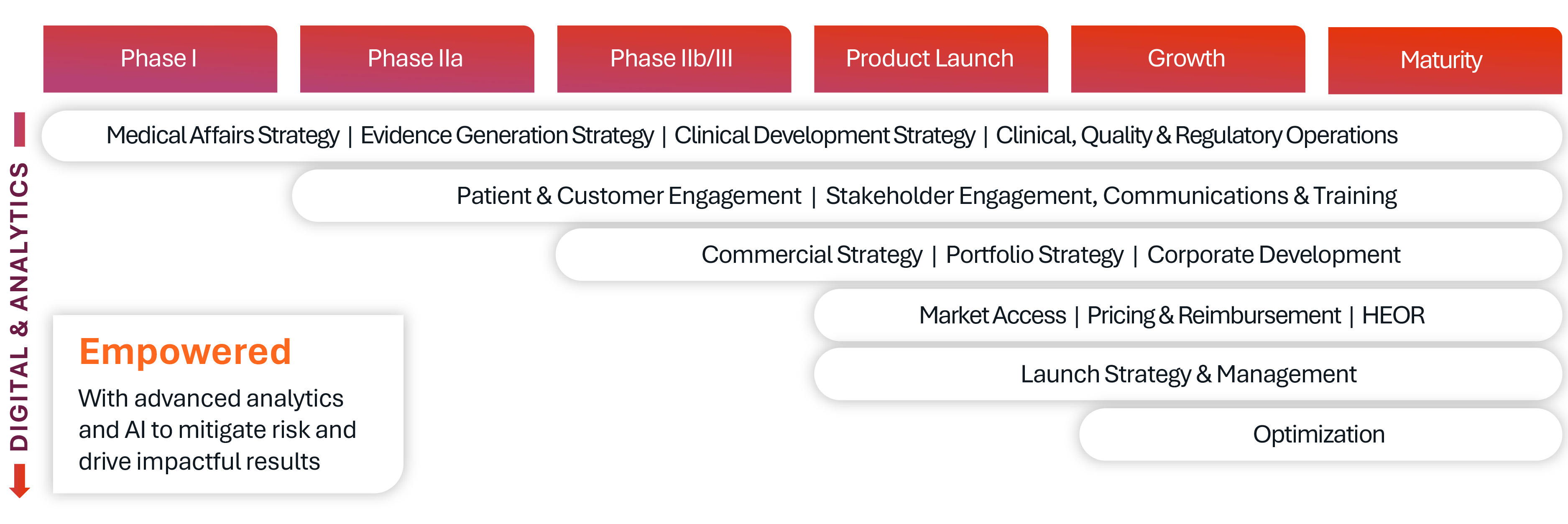
Making the right decisions early in the drug development journey is critical to success. Our team helps biopharmaceutical companies accelerate speed to market, reduce development risk, and enhance product differentiation through tailored product development strategy support.
We guide key decisions from early indication prioritization to clinical strategy and regulatory planning. Our approach is informed, systematic, and focused on value creation. We help clients prioritize indications by evaluating biology, feasibility, and commercial potential to ensure assets are positioned for success from the outset. We also support therapeutic differentiation and label strategy to increase patient benefit and drive commercial impact. Our teams design integrated clinical development plans that align with regulatory expectations and raise the probability of approval. To improve trial efficiency, we apply tools like Protocol Optimizer, which incorporates site and patient insights to address challenges such as tighter eligibility criteria, enrollment competition, and diversity and inclusion goals.
Drawing on deep preclinical, clinical, and commercial expertise, our integrated, multidisciplinary team delivers actionable, evidence-based recommendations that reflect the realities of today’s drug development landscape.
Biopharmaceutical companies – regardless of size - face a shared challenge: the future of the organization often hinges on a small number of pipeline assets, many targeting new markets where internal experience and brand equity may be limited. In these moments, partnering with a trusted expert can reduce the risk of a missed launch.
Our team – formerly Campbell Alliance – has nearly 25 years of experience in Commercialization and Launch Excellence. In the past five years alone, more than 145 biopharmaceutical companies have turned to us to guide their launch efforts, from early strategy through operational readiness to post-launch optimization.
What sets us apart is not just our heritage of innovation, but our unmatched depth of operational and therapeutic expertise. Our clients value our ability to navigate the complexities of their environments and the markets they serve. Our recent work spans more than 200 product and market types across six core therapeutic areas, including oncology, immunology, neurology, infectious disease, and musculoskeletal disease.
We also bring the full strength of Syneos Health to bear, drawing on a deep bench of experts from across our Clinical and Commercial Business Units to meet each client's unique needs.
In partnership with clients, our Medical Affairs consulting team develops and executes strategies that amplify science and value, driving impact from early development through commercialization and beyond.
With expertise in medical strategy and operations, field medical deployment, real-world late-phase research, integrated evidence, and scientific communications, we build integrated teams to optimize efficiency, create lasting and measurable value and help our clients communicate and educate their product’s safety, effectiveness, and impact on patient outcomes.
We are a best-in-class program management office (PMO) with services tailored to navigating the complexities of consortia management and to delivering impactful results that drive success in the competitive life sciences market.
Our team designs, develops and implements large-scale programs, broad transformation initiatives and global risk management strategies. We partner with clients to manage post-marketing commitments, support multi-sponsor engagements and provide grant management expertise.
To prepare our clients for regulatory and commercial challenges, our team delivers insights and event planning, including support for FDA advisory committee meetings and industry conferences.
Ensuring compliance across systems and projects is essential for success in the life sciences industry. Our Quality & Compliance team assists with both proactive planning and immediate responses to compliance needs. We facilitate global QA consistency, covering all auditing requirements, regulation types, and markets.
Our services include conducting comprehensive audits and inspection readiness activities, ensuring compliance with safety regulations, assessing and qualifying vendors, and performing REMS audits for regulatory compliance. We are committed to providing comprehensive quality and compliance support that drives success for our customers."

Organization Development
We partner with life sciences organizations, helping them navigate complexity and transformation, guiding products from discovery to launch and beyond. We operate at the intersection of technology, business and humanity, ensuring that our solutions are developed with a real-world mindset, fueled by global functional and therapeutic expertise.
- Organizational Design
- Operational Efficiency
- Program Management
- Digital Transformation
- Quality and Compliance

Global Impact
Collaboration across disciplines and teams enables us to develop holistic, integrated strategies that drive measurable results. By connecting insights across clinical development, commercialization and regulatory strategy, we provide a seamless approach to problem solving that breaks down silos and geographies. We leverage local expertise on a global footprint to help customers develop tailor-made market entry and commercialization strategies.

Digital Health, AI and Analytics Solutions
Our pharmaceutical consulting solutions are amplified by a dedicated team of digital health, AI, and analytics experts – powered by Kinetic, Syneos Health’s proprietary insights and performance platform. This team brings together advanced data science, machine learning and real-world evidence capabilities to inform smarter strategies and accelerate execution.
With a suite of proprietary tools, such as Brand Optimizer and the Market Dynamics Engine, we help clients uncover hidden opportunities, optimize decision making and stay ahead of industry shifts. It’s not just about having data – it’s about transforming it into clarity, foresight and measurable impact.
We partner with biopharma leaders to transform complexity into clarity and impact, enabling bold decision makers to navigate high-stakes challenges with confidence

Proudly recognized as a top ranked consulting firm across North America, APAC and EMEA in Vault’s 2026 rankings — reflecting the strength of our people and culture.

Recognized by Forbes as one of America’s Best Management Consulting for 10 consecutive years




