China: The New Pace Car of Change
2021 Health Trends
What was an untapped market mere years ago is now surging. The pharmaceutical and biotech market in China is experiencing the most dramatic growth in the world, with in-region innovation, increased approvals of novel drugs and maturing research and development capabilities. One key reason why: differences in regulations between the Western World and China allow researchers there to more quickly understand how a treatment is working and make modifications on shorter time frames. That’s making China a center for gene therapy and CAR-T medical discovery and clinical trials. Biotechs within China and pharma leaders around the world are doing research at scale in the region, effectively working with CFDA to get protocols approved within a year. There are complexities, of course, including building specialty labs in the region and training new trial sites. But, the benefits to patients are outsized: access to novel oncology medications before the rest of world.
Critical Question
As we enter 2021, it’s a footrace. Who will move fastest and get the most penetration: local biotech, out-of-region biotech, or large pharma who have scaled up investment in China for a decade?
Did you know...
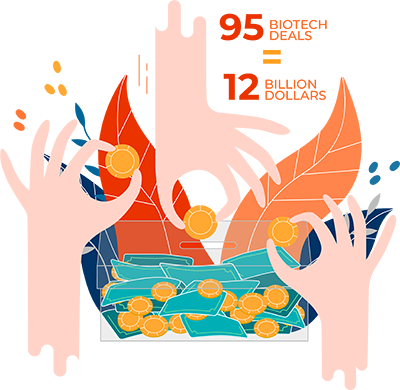

$12 Billion: Venture capital investment may have slowed overall in the face of the pandemic, but not in Chinese biotech. The first half of 2020 saw 95 biotech deals, worth $12 billion, an unprecedented amount of activity.
— Bioworld, 2020
54%: China leads the world in the number of active CAR-T trials with 54.2%. The U.S. is second with 38.5%, and the next largest trial centers come in with single digits (UK, 4.5%; France, 4.1%; Belgium, 3.6%).
— DRG, 2020

