Remote Care
2021 Health Trends

When the pandemic took hold, practices and research sites limited physical access to protect people while health systems and industry partners moved to rapidly scale digital and voice channels to maximize patient connectivity. Regulators and payers joined the cause to reduce friction and fuel reimbursement.
Even as that immediacy and urgency fade, healthcare leaders and patients alike have a changed expectation for a future that pivots off a connection, not an office or site. As we enter 2021, the rise, fall and reset of remote monitoring and commercial telemedicine will largely shape the future of care and drive how life sciences leaders engage and support patients and prescribers.
Critical Question
- What will the new best practices be for how industry can support patients and professionals connecting across distance?
- How can this new fluency consistently create access to trials for more patients and access to support for more patients?
Learn more: Download the deep dive trend report on Remote Care below.
Did You Know...
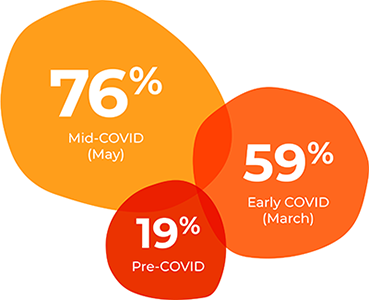
300%: The number of practices offering telemedicine connectivity in the U.S. grew from 59% to 76% at the height of the 2020 wave of the pandemic.
– Syneos Health, 2020

Family Teledoc: The healthcare division of Chinese e-commerce giant JD.com recently launched a new service package that looks more like a traditional primary care program. As many as eight family members share a family doctor package that includes 24/7 immediate medical support from GPs and specialist medical consults within 48 hours.
– MobiHealthNews, 2020

