The Race to Return Safe
2021 Health Trends

Our industry’s response to COVID-19 has changed the global conversation about the impact pharmaceutical development has on lives and families. Today, we’re seeing a resurgence in public faith in medical innovation as companies race to develop diagnostics, treatments and vaccines in what we all hope will be record time.
2021 represents a critical pivot point where life sciences either builds on this new awareness of discovery and clinical participation or fights through a battle of vaccine hesitancy. This year, we do the work to show the impact, safety, integrity and mission the industry has long been committed to. The longer-term impact is stronger relationships with the advocates, prescribers and patients we all serve.
Critical Question
How do we leverage this moment of industry impact to build enduring trust and a strong understanding of the need to participate in clinical development?
Sparks for the Race to Return Safe
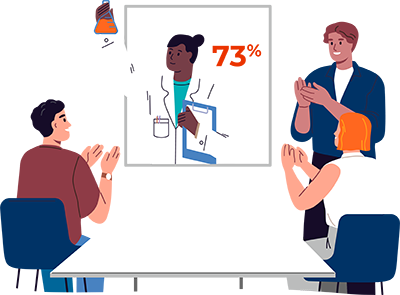
73%: In April, just as COVID-19 was dramatically changing behaviors and outlooks around the world, the annual Trust Barometer report fielded a spring update and found a record-breaking level of trust for the pharmaceutical industry, with the biggest increases in trust in Canada, Germany and the U.S.
- PM Live, 2020
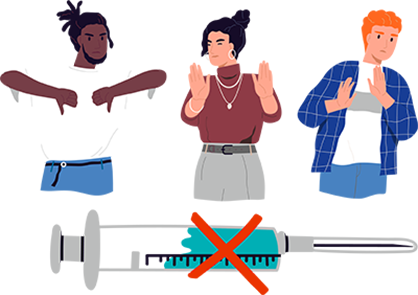
1:3 - As progress toward a cure advances, the debate about who will take it grows louder. In early fall polling, 35% of Americans said that they would not take a COVID-19 vaccine, even if the FDA approved and provided it at no cost. 1:6 people in the UK agreed, saying they are unlikely to or definitely will not get the vaccine.
— Gallup, 2020; Euronews, 2020

