Clinical Development (Full-Service and FSP CRO Solutions)
Turning potential into progress
In clinical development, timing is everything. That’s why Syneos Health®, a leading contract research organization and full-service clinical development partner, starts where others hesitate — with the end in mind.

Delivering solutions for today and strategies for what’s next
At Syneos Health, we’re inspired by what’s possible.
We take the time to truly understand what drives your program and makes it unique. Then we bring the right therapeutic, scientific and operational experts to the table to work with yours. They draw on knowledge from decades of experience and thousands of trials to provide clinical trial services that adapt to your needs and help you advance through every milestone with confidence.
By applying technology, predictive modeling and proprietary methodologies, we aim to increase study confidence, minimize risk and enable smarter, real-time decisions so that you’re ready for what’s next.
Expertise that moves medicines forward
Syneos Health by the Numbers:
40
years' clinical trial experience
480+
MDs
750+
PhDs with deep therapeutic, scientific and operational expertise
100+
years of combined advocacy and patient affairs experience
In the last five years, Syneos Health has helped to develop or commercialize:
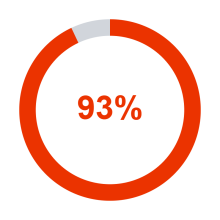
of novel drugs approved by the US Food and Drug Administration (FDA)

of products granted marketing
authorization by the European
Medicines Agency (EMA)
Comprehensive, insight-driven clinical trial services designed to meet your needs and help achieve your goal—getting life-changing medicines to patients faster
- Strategic Clinical Planning and Global Trial Execution: Offering full-service clinical development solutions across all phases including Early Phase, Phase II-IV and Real World Evidence
- Functional Service Provider (FSP 360): A customized outsourcing approach that prioritizes flexibility and fit-for-purpose solutions, aiming to ease the burden of resource management and functional delivery while enabling sponsor visibility and control of clinical development
- Site and Patient Access: Targeted outreach strategies and evidence-based retention protocols, powered by advanced digital recruitment technologies, to accelerate timelines, reduce burden and optimize trial outcomes
- Study Startup and Activation: Supporting all aspects of clinical trial regulatory and site startup operations, from site identification/feasibility and ethics/regulatory submissions, to site contracting and budget negotiations and clinical study regulatory submissions strategy
- Patient Advocacy and Engagement: Adaptable solutions that elevate patient needs and preferences to the center of planning and execution, addressing the real-world priorities, burdens and goals of patient communities and care partners
- Safety and Pharmacovigilance: Providing both standalone and fully integrated, innovative solutions to support compliant and accurate clinical trial and post-marketing safety surveillance across the globe
- Regulatory Affairs: A portfolio of integrated solutions for every stage, from clinical development to registration and lifecycle management, informed by a deep understanding of the regulatory landscape and years of experience in navigating complex regulatory environments
- Bioanalytical Services: State-of-the-art bioanalytical technologies and deep scientific expertise supporting complex method development, validation and sample analysis
- Decentralized and Hybrid Clinical Trials: Combines fully integrated clinical, RWE and commercial insights, agile technologies and operational excellence to bring clinical trials closer to the patient and help enhance patient accessibility, accelerate recruitment and retention, and increase data acquisition efficiencies
- Clinical Development Services: Whether you need one or more specific capabilities or seek a strategic functional service provider (FSP 360) partnership, we can help with a full range of services for:
- Biostatistics and Statistical Programming
- Clinical Data Management
- Clinical Monitoring
- Medical Writing
- Trial Master File Operations
- Rater Training
- Clinical Trial Investigator Management
Explore our therapeutic expertise

Why choose Syneos Health for clinical trial services?
Our ability to deliver is fueled by the power of agile partnership:
- We bring bold innovation and integrated clinical research and commercial solutions to help you rethink how programs are run, accelerate timelines and extract value across the full asset lifecycle.
- We are a leading CRO in critical therapeutic areas such as oncology and hematology, neuroscience, rare disease and dermatology.
- Approximately 70% of our portfolio is made up of biotech customers, so we understand their need for skillful, stream-lined decision-making and operational agility.
- Our teams combine therapeutic depth with operational rigor, delivering >95% milestone adherence while embedding advanced analytics and real-time risk detection to mitigate risks and keep studies on plan.
- Our FSP 360 models consistently help sponsors gain efficiencies, increase visibility and improve execution across every phase of the trial lifecycle, delivering >97% cross-partnership key performance indicators, >94% satisfaction and >90% retention in our top partnerships, proving our commitment to long-term engagement and performance.
Full-Service Outsourcing (FSO) centralizes most or all clinical trial functions with one CRO partner. The CRO manages all aspects of the study—from protocol design and site selection to monitoring, data management, regulatory submissions and final reporting—providing end-to-end services under one contract. This model provides a single point of accountability along with more streamlined operations, reduced internal burden and access to global expertise and networks.
Functional Service Provider (FSP) engagements traditionally are those in which the sponsor retains oversight and strategic control of the study, while the FSP provides expertise and staff in defined areas such as biostatistics, data management, clinical monitoring, medical writing, safety or regulatory support. Syneos Health’s FSP 360 model has redefined what this partnership looks like. We embed specialized, cross-functional teams within sponsor organizations while also providing intelligent infrastructure and global agility—all guided by strategic collaboration with sponsors.



